Feng: Difference between revisions
imported>Student221 No edit summary |
imported>Student221 |
||
| (88 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
== Introduction == | == Introduction == | ||
Cancer screening is a procedure that aims to detect cancer before any signs or symptoms of cancer arise; it is potentially useful for medical diagnosis because early detection of cancer significantly increases the chances for successful treatment [1]. For the case of oral cancer, screening is particularly important as survival does correlate with stage, making early diagnosis and treatment optimal for this disease [2]. Unfortunately, the diagnosis constantly | Cancer screening is a procedure that aims to detect cancer before any signs or symptoms of cancer arise; it is potentially useful for medical diagnosis because early detection of cancer significantly increases the chances for successful treatment [1]. For the case of oral cancer, screening is particularly important as survival does correlate with stage, making early diagnosis and treatment optimal for this disease [2]. Unfortunately, the diagnosis constantly relies on physical examination followed by biopsy confirmation, which could result in delay in diagnosis [3]. As such, more and more research are being conducted to develop easy-to-use, cost effective oral cancer screening devices to encourage and improve the screening process. | ||
Currently, there is a number of devices available on the market for oral cancer screening purpose. Some examples are [https://apteryx.com/velscope/ Velscope®], [https://www.denmat.com/vizilite-pro-oral-lesion-screening-system.html ViziLite®] and [http://www.identafi.net/ Identafi®]. This project is based on the newly developed "OralEye" system from [https://www.fengyunvision.com/cn/products/#prodcut_id5 Fengyun Vision Technology], and aims to improve the overall usability, accuracy and efficiency of the system for oral screening. | Currently, there is a number of devices available on the market for oral cancer screening purpose. Some examples are [https://apteryx.com/velscope/ Velscope®], [https://www.denmat.com/vizilite-pro-oral-lesion-screening-system.html ViziLite®] and [http://www.identafi.net/ Identafi®]. This project is based on the newly developed "OralEye" system from [https://www.fengyunvision.com/cn/products/#prodcut_id5 Fengyun Vision Technology], and aims to improve the overall usability, accuracy and efficiency of the system for oral screening. | ||
| Line 10: | Line 10: | ||
[[File:Velscope_exam.png|200px|thumb|right|alt=Alt text|Figure 1: Oral examination using a Velscope® device. Source: https://apteryx.com/velscope/.]] | [[File:Velscope_exam.png|200px|thumb|right|alt=Alt text|Figure 1: Oral examination using a Velscope® device. Source: https://apteryx.com/velscope/.]] | ||
VELscope® is a hand held device that emits 400–460 nm wavelength light to excite auto-fluorescence from fluorophores in the mouth. Some researchers suggest that 405 nm wavelength light | VELscope® is a hand held device that emits 400–460 nm wavelength light to excite auto-fluorescence from fluorophores in the mouth. Some researchers suggest that 405 nm wavelength light is able to discriminate neoplastic and non-neoplastic tissue with high sensitivity and specificity [5]. However, there has also been a number of criticisms on VELscope® for the limited capacity to extend its use in general dentistry. Researchers are still trying to improve its specificity to allow wider clinical use [4]. | ||
Identafi® is a probe like device designed for multi-spectral screening of oral disease. It emits three types of light: white, violet (405 nm) and green-amber (545 nm) light. The white light provides classical visual inspection and the violet light | Identafi® is a probe like device designed for multi-spectral screening of oral disease. It emits three types of light: white, violet (405 nm) and green-amber (545 nm) light. The white light provides classical visual inspection and the violet light serves similar purpose as in VELscope®. The green-amber light, through reflection spectroscopy, is used to excite haemoglobin molecules in the blood, in order to visualise the vasculature [6]. Researchers found that the use of Itentafi provides more data that conventional oral exam. However, it requires high level of clinical training in oral pathology to interpret the results, and thus limiting its usage in general practice [7]. | ||
According to ref [4], a main limiting factor for universal usage of | According to ref [4], a main limiting factor for universal usage of the aforementioned devices is no demonstrated superiority compared to conventional oral exam. However, the authors acknowledge the possibility that these devices can be enhanced with "new approaches used to analyze optical imaging data". As such, this project aims to explore new methods towards easy-to-use oral florescence imaging system as well as accurate data interpretation to address the needs in oral cancer screening. | ||
== Methods == | == Methods == | ||
[[File:OralEyeCamera.png|200px|thumb|right|alt=Alt text|Figure 2: OralEye camera.]] | [[File:OralEyeCamera.png|200px|thumb|right|alt=Alt text|Figure 2: OralEye camera.]] | ||
The OralEye camera has a form factor similar to that of a torch or flashlight. | The OralEye camera has a form factor similar to that of a torch or flashlight, and is mounted on an optical post to take images of subjects or calibration targets. The camera is connected to a PC using a USB cable. The communication between the camera and PC is based on the Ethernet-over-USB protocol RNDIS. A software development kit (SDK) provided by Fengyun Vision was used to develop custom camera interfacing software. A basic illustration of the communication between OralEye and the PC is shown in Figure 3. | ||
[[File:Communication. | [[File:Communication.PNG|upright=2|thumb|left|Figure 3: OralEye communication protocol.]] | ||
The command line camera control software OralEyeCamera.exe is developed in Visual C++ with Visual Studio 2017. Most of the communication between the camera and PC is based on the TCP protocol. As such, the Winsock library is heavily used. The executable can take four types of input parameters to carry out different tasks. The syntax for using the command line tool is shown below: | |||
<code> | |||
OralEyeCamera.exe printParam | |||
OralEyeCamera.exe lightBlue|lightWhite|lightOff | |||
OralEyeCamera.exe blueShoot|whiteShoot|singleShoot [expTime] | |||
OralEyeCamera.exe task.json | |||
</code> | |||
The JSON file is used to specify a sequence of captures with different light/exposure settings. Its format is defined by the separate MATLAB software "oraleye". Comparing to invoking the executable in single shot mode, the consecutive image capture is optimized in terms of overhead in server and parameter setup. | |||
== Results == | |||
[[File:Subject003.png|thumb|right|upright=1.5|alt=Alt text|Figure 4: Subject 003 with white and blue illumination.]] | |||
With the OralEyeCamera.exe command line tool, we were able to capture sequences of frames with different lighting/exposure at a frame rate of about 2 fps. The frame rate is mainly limited by the overhead of file I/O, establishing socket communication and parameter setting for each frame. However, for a consecutive capture of 6 frames, the subject only needs to keep the mouth open for ~3 seconds, which is still feasible in practice. | |||
[[File:Subject001.png|thumb|upright=1.5|left|alt=Alt text|Figure 5: Subject 001 with white and blue illumination.]] | |||
[[File:Subject002.png|thumb|right|upright=1.5|alt=Alt text|Figure 6: Subject 002 with white and blue illumination.]] | |||
A pair of images of subject 003 are shown in Figure 4. In these images we can clearly see a number of fluorescence signatures, such as the red stripes and turquoise colored background on the tongue, which could potentially be the fluorescence of different bacteria [8]. There are also strong fluorescence spots over the lip of the subject, which could be related to the hair follicles [9] according to their shape and spacing. These signatures indicate that using color CMOS camera is an effective way to detect different types of fluorescence. With the spectral response curves of the camera sensor, it is also possible to locate the wavelength range of the relevant fluorescence. | |||
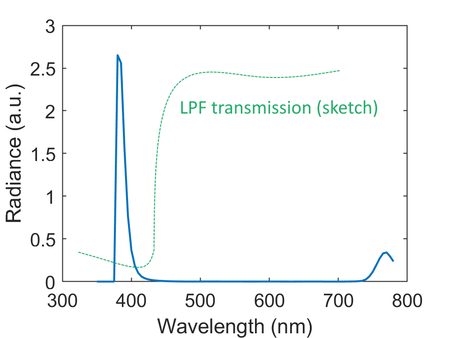
[[File:Oraleyespectrum_with_lpf.png|thumb|left|upright=1.5|alt=Alt text|Figure 7: OralEye blue LED spectrum and long pass filter (LPF) transmisivity]] | |||
However, the images also exposed a couple of design issues within the OralEye camera. Considering the subject images in Figure 5 and 6, there are much less visible fluorescence features comparing to Figure 4. Meanwhile, the blue light image is very much like the white light image with a global tint (because of the low color rendering index of the blue LED). This indicates there is light signal seen by all three channels of the camera sensor, which isn't supposed to occur from the initial design point of view. Moreover, in Figure 6, the chin rest shows strong saturated blue light reflection, which is also not supposed to occur because of the presence of a long pass filter (LPF) in front of the camera lens. | |||
In order to find out the underlying issue with the camera design, a measured OralEye blue LED spectrum along with a sketched long pass filter transmissivity are shown in Figure 7. It can be seen that the blue LED actually emits a significant amount of infrared radiation above 700 nm, which could potentially result from deep level recombination depending on the specific type of LED material [10]. Because the camera only has a long pass filter with no IR filtering capability, this portion of light is fully visible by all three channels of common CMOS sensors, whose color filters typically don't cover the IR band [11]. Moreover, the LPF has a gradual transition band, and the transmissivity in the stop band doesn't really goes to zero. This behavior allows some amount of incident blue light to make it through the LPF and reach the camera sensor. Moreover, comparing Figure 5 and 6, it is clear that there's mismatch between the working distances of the camera lens and the LED illuminator. In Figure 5, the central part of the tongue is at the sharpest focus, however, the illumination shows a dark, under illuminated region in that area. On the other hand, in Figure 6, the overall illumination is uniform and bright on the tongue, but the subject is strongly out of focus. These issues need to be addressed in the next generation of the system to produce more useful image data. | |||
== Conclusions == | |||
It is clear that auto fluorescence imaging is promising for examining the over all health of the oral cavity. However, current oral screening devices all have their own limitations which prevent wide usage in practice. In this project, we developed the software infrastructure for interfacing with the OralEye camera, and collected fluorescence image data to examine its efficacy. Through detailed analysis, we conclude that OralEye camera could potentially be useful in fast examination and image acquisition for oral screening purpose. However, there are several issues with the current system design that need to be addressed before it becomes suitable for clinical test. | |||
== Appendix == | |||
The source code of OralEyeCamera.exe is available at https://github.com/akahs/OralEyeCamera. | |||
== References == | == References == | ||
[1] https://www.who.int/cancer/detection/en/ | [1] https://www.who.int/cancer/detection/en/ | ||
[2] Ries LAG, Kosary CL, Hankey BF, et al. SEER Cancer Statistics Review, 1973-1995. Bethesda, MD, NCI.2. American Cancer Society, Facts and Figures, 2000. | [2] Ries LAG, Kosary CL, Hankey BF, et al. ''SEER Cancer Statistics Review'', 1973-1995. Bethesda, MD, NCI.2. American Cancer Society, Facts and Figures, 2000. | ||
[3] https://oralcancerfoundation.org/discovery-diagnosis/cancer-screening-protocols/ | [3] https://oralcancerfoundation.org/discovery-diagnosis/cancer-screening-protocols/ | ||
| Line 46: | Line 65: | ||
[6] Messadi, D. V., et al. "The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions", ''Int. J. Oral Sci.'' '''6''', 162–167 (2014) | [6] Messadi, D. V., et al. "The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions", ''Int. J. Oral Sci.'' '''6''', 162–167 (2014) | ||
[7] Lalla, Y., et al. "Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy", ''J. Am. Dent. Assoc.'' '''147''', 650–660. | [7] Lalla, Y., et al. "Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy", ''J. Am. Dent. Assoc.'' '''147''', 650–660 (2016) | ||
[8] Kang, S., et al. "Fluorescence fingerprints of oral bacteria", ''J. Biophotonics'', e201900190 (2019) | |||
[9] Li, L., et al. "Nestin expression in hair follicle sheath progenitor cells", ''Proc. Natl. Acad. Sci.'' '''100''', 9958 (2003) | |||
[10] Lim, et al. "UV Electroluminescence Emission from ZnO Light-Emitting Diodes Grown by High-Temperature Radiofrequency Sputtering" ''Adv. Mater.'' '''18''', 2720 (2006) | |||
[11] Dworak, V., et al. "Strategy for the Development of a Smart NDVI Camera System for Outdoor Plant Detection and Agricultural Embedded Systems", ''Sensors'', '''13''', 1523 (2013) | |||
Latest revision as of 06:49, 14 December 2019
Introduction
Cancer screening is a procedure that aims to detect cancer before any signs or symptoms of cancer arise; it is potentially useful for medical diagnosis because early detection of cancer significantly increases the chances for successful treatment [1]. For the case of oral cancer, screening is particularly important as survival does correlate with stage, making early diagnosis and treatment optimal for this disease [2]. Unfortunately, the diagnosis constantly relies on physical examination followed by biopsy confirmation, which could result in delay in diagnosis [3]. As such, more and more research are being conducted to develop easy-to-use, cost effective oral cancer screening devices to encourage and improve the screening process.
Currently, there is a number of devices available on the market for oral cancer screening purpose. Some examples are Velscope®, ViziLite® and Identafi®. This project is based on the newly developed "OralEye" system from Fengyun Vision Technology, and aims to improve the overall usability, accuracy and efficiency of the system for oral screening.
Background
This section provides an overview of existing oral cancer screening devices as well as areas of improvements proposed by researchers, which will be serving as guidance for development of the new screening system based on OralEye camera.
ViziLite is a disposable capsule like device that relies on chemiluminescence in 430-580 nm. Although researchers found its potential utility in identifying occult epithelial abnormalities, ViziLite suffers from high false positive and negative levels, and thus limiting its clinical application [4].
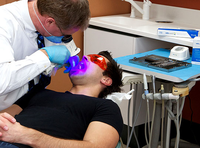
VELscope® is a hand held device that emits 400–460 nm wavelength light to excite auto-fluorescence from fluorophores in the mouth. Some researchers suggest that 405 nm wavelength light is able to discriminate neoplastic and non-neoplastic tissue with high sensitivity and specificity [5]. However, there has also been a number of criticisms on VELscope® for the limited capacity to extend its use in general dentistry. Researchers are still trying to improve its specificity to allow wider clinical use [4].
Identafi® is a probe like device designed for multi-spectral screening of oral disease. It emits three types of light: white, violet (405 nm) and green-amber (545 nm) light. The white light provides classical visual inspection and the violet light serves similar purpose as in VELscope®. The green-amber light, through reflection spectroscopy, is used to excite haemoglobin molecules in the blood, in order to visualise the vasculature [6]. Researchers found that the use of Itentafi provides more data that conventional oral exam. However, it requires high level of clinical training in oral pathology to interpret the results, and thus limiting its usage in general practice [7].
According to ref [4], a main limiting factor for universal usage of the aforementioned devices is no demonstrated superiority compared to conventional oral exam. However, the authors acknowledge the possibility that these devices can be enhanced with "new approaches used to analyze optical imaging data". As such, this project aims to explore new methods towards easy-to-use oral florescence imaging system as well as accurate data interpretation to address the needs in oral cancer screening.
Methods

The OralEye camera has a form factor similar to that of a torch or flashlight, and is mounted on an optical post to take images of subjects or calibration targets. The camera is connected to a PC using a USB cable. The communication between the camera and PC is based on the Ethernet-over-USB protocol RNDIS. A software development kit (SDK) provided by Fengyun Vision was used to develop custom camera interfacing software. A basic illustration of the communication between OralEye and the PC is shown in Figure 3.

The command line camera control software OralEyeCamera.exe is developed in Visual C++ with Visual Studio 2017. Most of the communication between the camera and PC is based on the TCP protocol. As such, the Winsock library is heavily used. The executable can take four types of input parameters to carry out different tasks. The syntax for using the command line tool is shown below:
OralEyeCamera.exe printParam
OralEyeCamera.exe lightBlue|lightWhite|lightOff
OralEyeCamera.exe blueShoot|whiteShoot|singleShoot [expTime]
OralEyeCamera.exe task.json
The JSON file is used to specify a sequence of captures with different light/exposure settings. Its format is defined by the separate MATLAB software "oraleye". Comparing to invoking the executable in single shot mode, the consecutive image capture is optimized in terms of overhead in server and parameter setup.
Results
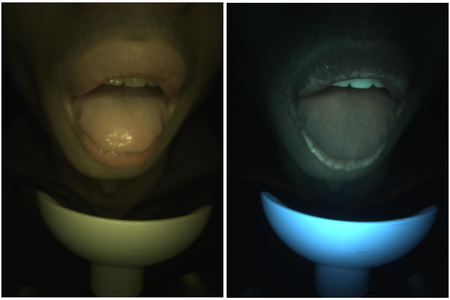
With the OralEyeCamera.exe command line tool, we were able to capture sequences of frames with different lighting/exposure at a frame rate of about 2 fps. The frame rate is mainly limited by the overhead of file I/O, establishing socket communication and parameter setting for each frame. However, for a consecutive capture of 6 frames, the subject only needs to keep the mouth open for ~3 seconds, which is still feasible in practice.
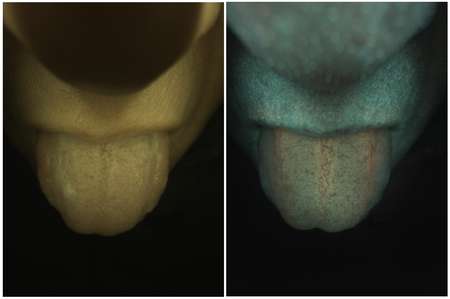
A pair of images of subject 003 are shown in Figure 4. In these images we can clearly see a number of fluorescence signatures, such as the red stripes and turquoise colored background on the tongue, which could potentially be the fluorescence of different bacteria [8]. There are also strong fluorescence spots over the lip of the subject, which could be related to the hair follicles [9] according to their shape and spacing. These signatures indicate that using color CMOS camera is an effective way to detect different types of fluorescence. With the spectral response curves of the camera sensor, it is also possible to locate the wavelength range of the relevant fluorescence.
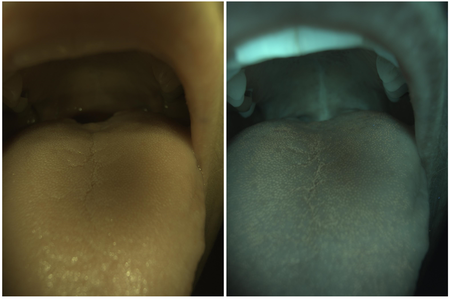
However, the images also exposed a couple of design issues within the OralEye camera. Considering the subject images in Figure 5 and 6, there are much less visible fluorescence features comparing to Figure 4. Meanwhile, the blue light image is very much like the white light image with a global tint (because of the low color rendering index of the blue LED). This indicates there is light signal seen by all three channels of the camera sensor, which isn't supposed to occur from the initial design point of view. Moreover, in Figure 6, the chin rest shows strong saturated blue light reflection, which is also not supposed to occur because of the presence of a long pass filter (LPF) in front of the camera lens.
In order to find out the underlying issue with the camera design, a measured OralEye blue LED spectrum along with a sketched long pass filter transmissivity are shown in Figure 7. It can be seen that the blue LED actually emits a significant amount of infrared radiation above 700 nm, which could potentially result from deep level recombination depending on the specific type of LED material [10]. Because the camera only has a long pass filter with no IR filtering capability, this portion of light is fully visible by all three channels of common CMOS sensors, whose color filters typically don't cover the IR band [11]. Moreover, the LPF has a gradual transition band, and the transmissivity in the stop band doesn't really goes to zero. This behavior allows some amount of incident blue light to make it through the LPF and reach the camera sensor. Moreover, comparing Figure 5 and 6, it is clear that there's mismatch between the working distances of the camera lens and the LED illuminator. In Figure 5, the central part of the tongue is at the sharpest focus, however, the illumination shows a dark, under illuminated region in that area. On the other hand, in Figure 6, the overall illumination is uniform and bright on the tongue, but the subject is strongly out of focus. These issues need to be addressed in the next generation of the system to produce more useful image data.
Conclusions
It is clear that auto fluorescence imaging is promising for examining the over all health of the oral cavity. However, current oral screening devices all have their own limitations which prevent wide usage in practice. In this project, we developed the software infrastructure for interfacing with the OralEye camera, and collected fluorescence image data to examine its efficacy. Through detailed analysis, we conclude that OralEye camera could potentially be useful in fast examination and image acquisition for oral screening purpose. However, there are several issues with the current system design that need to be addressed before it becomes suitable for clinical test.
Appendix
The source code of OralEyeCamera.exe is available at https://github.com/akahs/OralEyeCamera.
References
[1] https://www.who.int/cancer/detection/en/
[2] Ries LAG, Kosary CL, Hankey BF, et al. SEER Cancer Statistics Review, 1973-1995. Bethesda, MD, NCI.2. American Cancer Society, Facts and Figures, 2000.
[3] https://oralcancerfoundation.org/discovery-diagnosis/cancer-screening-protocols/
[4] Mascitti, M., et al. "An Overview on Current Non-invasive Diagnostic Devices in Oral Oncology" Front. Physiol. 9, 1510 (2018)
[5] Roblyer, D., et al. "Objective detection and delineation of oral neoplasia using autofluorescence imaging", Cancer Prev. Res. 2, 423–431 (2009)
[6] Messadi, D. V., et al. "The clinical effectiveness of reflectance optical spectroscopy for the in vivo diagnosis of oral lesions", Int. J. Oral Sci. 6, 162–167 (2014)
[7] Lalla, Y., et al. "Assessment of oral mucosal lesions with autofluorescence imaging and reflectance spectroscopy", J. Am. Dent. Assoc. 147, 650–660 (2016)
[8] Kang, S., et al. "Fluorescence fingerprints of oral bacteria", J. Biophotonics, e201900190 (2019)
[9] Li, L., et al. "Nestin expression in hair follicle sheath progenitor cells", Proc. Natl. Acad. Sci. 100, 9958 (2003)
[10] Lim, et al. "UV Electroluminescence Emission from ZnO Light-Emitting Diodes Grown by High-Temperature Radiofrequency Sputtering" Adv. Mater. 18, 2720 (2006)
[11] Dworak, V., et al. "Strategy for the Development of a Smart NDVI Camera System for Outdoor Plant Detection and Agricultural Embedded Systems", Sensors, 13, 1523 (2013)