Samir
Investigating the Effects of Smoothing and ICA on High-Resolution fMRI Data
Summary
This project explores how two popular preprocessing methods, smoothing and ICA, influence high resolution fMRI datasets. Using a dataset focused on the brain's motor regions, the motor cortex, the basal ganglia and the cerebellum, I investigated:
1. The effect of smoothing on T-statistics and voxel significance
2. The ability of ICA to delineate task-specific signals from noise
Motivation
The ability to coordinate muscles is fundamental to human motor control, and underlies even simple motions like holding a cup with a hand and flipping a switch with the same arm’s elbow. Past research has not elucidated the brain’s coordination strategy because neurophysiology experiments are confounded by the fact that at a fine spatial scale, motor related neurons correlate with all observable movement parameters [cite] , while at a higher spatial scale, functional neuroimaging experiments have been unable to delineate any generalizable motor organization apart from fractured somatotopic sensorimotor maps [cite].
Probing the motor control regions in a manner that elucidates the structure of the underlying motor controller thus requires novel experiments. High resolution fMRI is ideal for such experiments since it can probe neural correlates of motor activity with a high spatial resolution (1.5mm3 voxels) and a temporal resolution similar to most motor tasks (a few seconds). The same high resolution fMRI data, however, might be interpreted in multiple ways with minor changes to the processing pipeline. The goal of this project is to understand how two popular preprocessing steps, smoothing and ICA, can influence statistical results.
Methods
A novel motor control dataset was acquired for this study. The data collection started before the course but continued through its duration.
Subject Details
For the data presented in this project, I am including scans for 3 healthy right-handed volunteers (2 males; 19–28 yr of age) from our set of subjects. All subjects were informed about the experiment's details in advance, and gave their informed written consent to a protocol approved by the Institutional Review Board of Stanford University. The subjects were healthy and did not have any psychiatric or neurological disorders at the time or in the past. Subjects were asked to lie supine on the scanner bed, and their heads were surrounded by cloth to comfort and reduce head movements. In addition, they bit down on a bite bar for the duration of the study. The bite bar was customized to each subject's dental structure with a slow-hardening putty. Our controls removed motion related artifacts in almost all our datasets (Fig. 1 shows a representative example).
Task Details
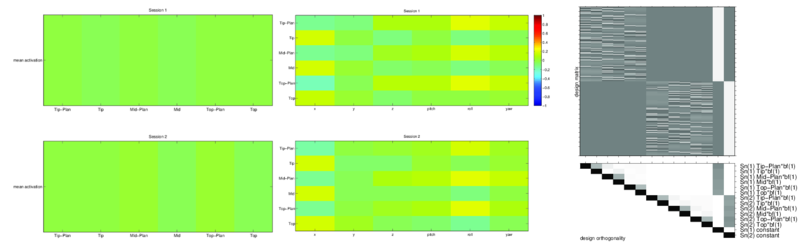
Subjects were asked to perform writing tasks like drawing a square with a pencil. They were provided visual text stimuli indicating when to plan and execute motions, and when to rest. For instance, one stimulus sequence would be: `Plan : Square' (yellow, 5 sec), `Execute : Square' (green, 8 sec), and `Rest' (red, 3-11 sec, randomized). During the plan phase subjects would plan their motion and possibly adjust grasp position, but would not make any whole arm motions. During the execute phase, subjects would move their entire arm to draw a square, without any finger movement. And during the rest phase, they would place their arm on their torso and rest. Subjects were asked to abandon tasks midway if they could not complete them in time.
The task presentation was randomized and orthogonalized using optseq (http://surfer.nmr.mgh.harvard.edu/optseq/). A sample design matrix representing one subject's task presentation demonstrates optseq's sequencing (Fig. 1).
Stimulus Presentation
The task description text was displayed on a modified Samsung SyncMaster 305T 30 inch diagonal display (76 cm, 16:10 aspect ratio), built by Resonance Technology (www.mrivideo.com) at a resolution of 1280x800 pixels. Subjects viewed the display through a double mirror at a distance of about 190cm from the head-coil, and the display was inverted so text appeared normal to the subjects. The text was rendered on the screen using VisionEgg, a freely available python based stimulus presentation software.
Scan Sequence Details
We acquired images GE MR 750 3-Tesla scanner and a Nova 32 channel head coil at the Stanford Center for Cognitive and Neurobiological Imaging (CNI). Two series of 510 functional volumes were acquired using a gradient echo, echoplanar sequence with a 134 square matrix, 27 oblique slices, 2mm thick, with a 0.5mm gap between slices. The voxel size was 1.5mm x 1.5mm x 2.0mm, repetition time was 2 sec, echo time was 33ms and flip angle was 75degrees. The slices were adjusted for each subject to include cerebellum, posterior striatum, and motor cortex.
The functional images were overlaid on a co-aligned, high-resolution anatomical scan of the whole brain taken at the end of each session (BRAVO sequence; TR = 7.8 sec; TE = 3.1 ms, flip angle = 12 degrees; matrix, 300x300; 0.8 mm, isotropic).
The Effect of Smoothing
Data

Discussion
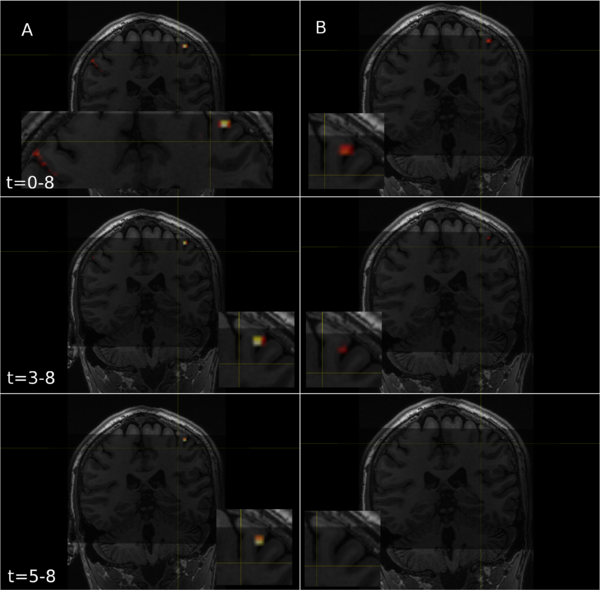
For the high-resolution data we collected, smoothing substantially decreased the T-statistic across the dataset, and often led to a complete loss of significantly activated voxels in entire brain regions (Fig. 2). While smoothing theoretically increases signal to noise, it did not help our analysis much. The only metric where smoothing held a minor advantage, was that it avoided false positives throughout the data (Fig. 3).
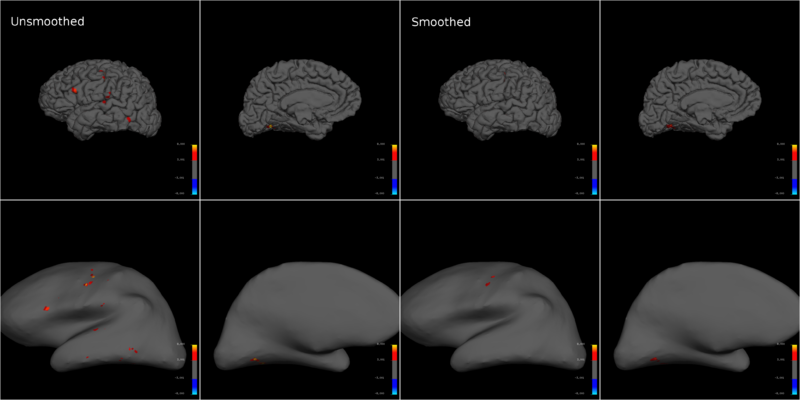
I reconstructed each individual subject's brain surfaces to avoid introducing co-registration and normalization related errors, and created inflated and pial brain models with freesurfer. The projected T-statistics for a subject demonstrate how smoothing dramatically reduced the significance of motor activity (Fig. 4). This effect was conserved across subjects and multiple motion types. Note that projecting on to an inflated brain warps single voxels into circles, making them seem larger than they truly are.
Another important aspect to note is that while anatomical and smoothed functional underlays might display grey matter, CSF related scanner artifacts could have actually created voids at the same position. However, if activation in the void persists after carefully checking the data for motion artifacts and using family-wide error correction you can't reject it. The vasculature is often dense at the boundaries of the cortical surface and if the neural activation in neighboring regions is strongly correlated with the task, then the blood flow in the vasculature might make it seem like the BOLD signal is coming from the artifact region [Personal communications with Prof. Gary Glover, Stanford University].
Data
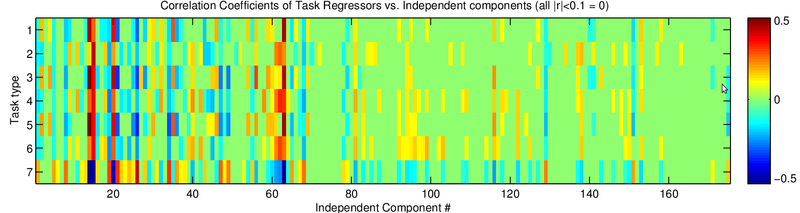
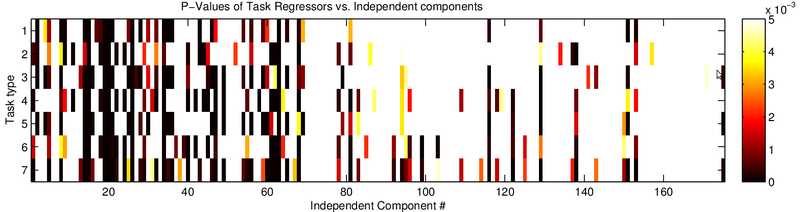
Discussion
Since smoothing did not seem like a valuable strategy to increase the contrast significance, I next explored whether ICA can delineate noise from task-related activity.
Unsmoothed Neural Correlates of Writing Tasks
Data
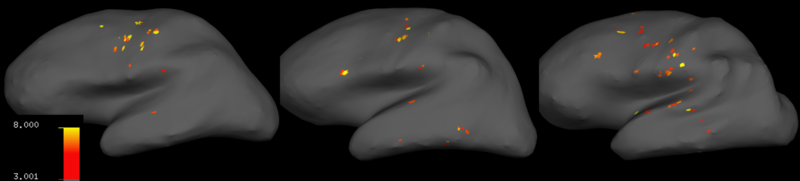
Discussion
With my results, I decided to process my data without smoothing it. The t-statistic maps for three subjects measuring motor activity during writing displayed significant neural correlates of writing in motor, pre-motor and somatosensory cortices (Fig. 5), as well as cerebellum (not shown). The locations at which voxels correlated significantly with task regressors changed from subject to subject, whose brains had visibly different shapes.
Conclusions and Future Work
Appendix
Presentation
Script
REFERENCES
[1]