Rav: Difference between revisions
imported>Psych204B No edit summary |
imported>Psych204B No edit summary |
||
| (17 intermediate revisions by the same user not shown) | |||
| Line 18: | Line 18: | ||
== Experiment Structure == | == Experiment Structure == | ||
60 IAPS pictures (images with affective content) were split into 30 pairs. Within a pair, one picture was designated as Picture 1 and the other as Picture 2. Picture 1 and Picture 2 were counterbalanced for valence and arousal (IAPS population ratings). In the fMRI, subjects were shown Picture 1 for 500ms followed by Picture 2 for 500ms. They were asked to make a choice between the two pictures. The chosen picture was displayed for 4s—a duration long enough to ensure that the preferred picture was viewed and processed; this attached real affective consequences to each choice. | 60 IAPS pictures (images with affective content) were split into 30 pairs. Within a pair, one picture was designated as Picture 1 and the other as Picture 2. Picture 1 and Picture 2 were counterbalanced for valence and arousal (IAPS population ratings). | ||
In the fMRI, subjects were shown Picture 1 for 500ms followed by Picture 2 for 500ms. They were asked to make a choice between the two pictures. The chosen picture was displayed for 4s—a duration long enough to ensure that the preferred picture was viewed and processed; this attached real affective consequences to each choice. | |||
Outside the fMRI, subjects were asked to rate each image on a 1-7 scale for valence and arousal | |||
== Hypothesis == | == Hypothesis == | ||
We | We expected to see 2 sets of results: | ||
1) Distinct areas of the brain would be active for high vs. low valence pictures and for high vs. low arousal pictures. Since a variety of independent sources confirm that valence and arousal are core dimensions of affect, finding neural bases that underlie these dimensions is central to the understanding of affect. | |||
= Methods = | 2) We expect activations in these regions to drive choice. In particular a greater positive arousal (PA) would predict choice in cases where both pictures have PA > 0. Similarly in cases where NA > 0, we expect that lower NA would predict choice. | ||
== | |||
= fMRI Methods = | |||
== Expected Bases of A/V == | |||
Based on prior work in the Knutson Lab [http://www-psych.stanford.edu/~span/Publications/bk08prsb.pdf] we expected particular sub-cortical areas to show differential activation: | |||
For Positive Arousal (PA) we expected to see some activation in the Ventral Striatum (Nucleus Accumbens) in particular. | |||
For Negative Arousal (NA) we expected to see some activation in the Insula. | |||
In addition we expected to see some activation in the mPFC for both high valence and arousal pictures. | |||
=== Subjects === | === Subjects === | ||
Subjects were 5 healthy volunteers. | Subjects were 5 healthy volunteers. Results presented here are for a single subject (myself) | ||
=== MR acquisition === | === MR acquisition === | ||
Data were obtained on | Data were obtained on the CNI scanner at Stanford University. Functional and Anatomicals were reconstructed using CNI algorithms. | ||
AFNI was used to process and analyze the data. | |||
=== Trial Structure & Shaving of Leading TRs === | |||
In this experiment we used the following presentation schedule: | |||
Pic 1: 500MS followed by fixation for 500MS | |||
Pic 2: 500MS followed by fixation for 500MS | |||
Consider Your Choice: 2s | |||
Make Your Selection: 2s | |||
Variable ITI: 2/4/6s | |||
Due to this Trial Structure we chose at TR = 1s. | |||
Further we chose to have 8 leading and 8 trialing TRs to adjust for fMRI start up effects. We eliminated these TRs prior to pre-processing. | |||
= | === Pre-processing === | ||
All data were motion corrected, smoothed, drift corrected, normalized and warped. | |||
== | ==== Motion Corrected ==== | ||
The AFNI command '''3dvolreg''' was used for motion correction. | |||
== | ==== Smoothing ==== | ||
A blurring kernel of 4mm was used. | |||
== | ==== Drift Correction ==== | ||
A Fourier highpass filter of 0.011 was used to drift-correct | |||
Additionally: | |||
Data was normalized to percent signal change | |||
Anatomical and functional scans were wrapped to talairach space | |||
=== Post-processing === | |||
We used regressors and self ratings to do the analysis. | |||
First we created contrasts (1 0 or 1 0 -1 or parametric) that described the effects. | |||
1,0 -> picture on, picture off | |||
1,0,-1 -> high valence picture, no picture, low valence picture (similarly for arousal) | |||
3,2,1,0,-1,-2,-3 --> exact ratings of valence/arousal deviating from mean 0 | |||
= | Next we convolved the contrasts with the hemodynamic response function | ||
Finally we took the normalized functional data and ran a regression with the contrasts (as long as all contrasts are orthogonal, or aka non-covariance between contrasts). We examined the contrasts in AFNI | |||
= Results = | |||
== Valence == | |||
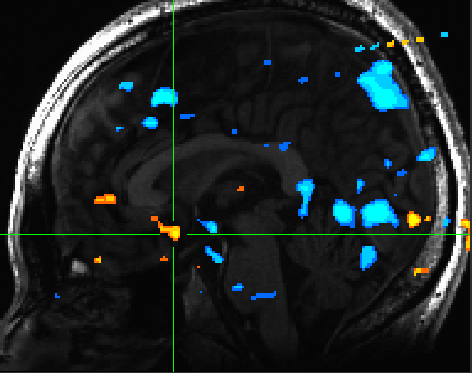
The ventral striatum was reliably activated for positively valenced pictures compared to negatively valenced pictures (Figure 2) | |||
[[File:valence_nacc.jpg | Figure 2]] | |||
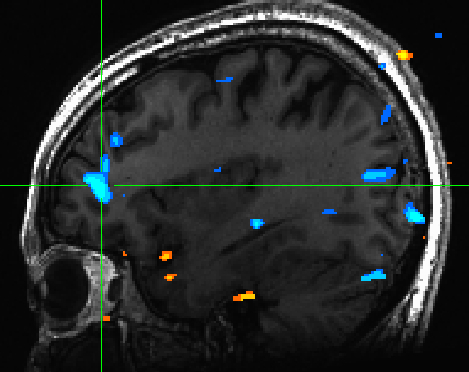
We also saw de-activation in the mPFC for highly valenced pictures -- indicating that low valenced pictures are cognitively processed to a greater extent than high valenced pictures (Figure 3) | |||
[[File:valence_mpfc.jpg | Figure 3]] | |||
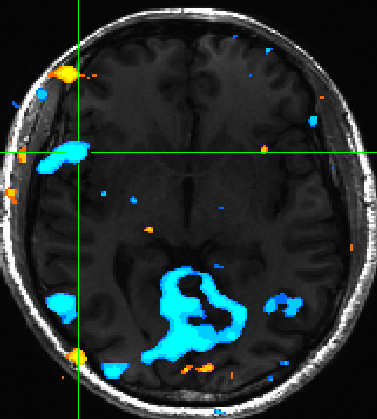
Expectedly we also observed insula activation for negatively valenced pictures (Figure 4) | |||
[[File:valence_insula.jpg | Figure 4]] | |||
== Arousal == | |||
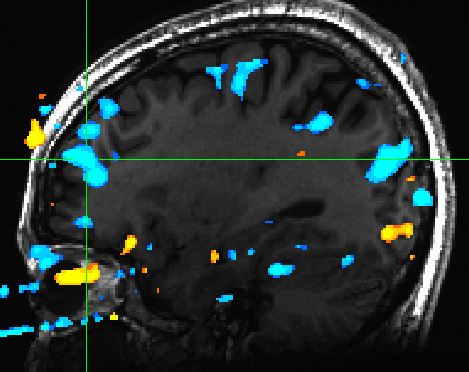
There were no reliable sub-cortical activations for arousal. However we did observe PFC and mPFC activations for low arousal pictures. (Figure 5) | |||
[[File:arousal_pfc_mpfc.jpg | Figure 5]] | |||
= | == Choice == | ||
We do observe that images with higher NAcc activation are chosen over images with lower NAcc activation. | |||
Similarly, negative images with lower insula activation are chosen over images with higher insula activation. | |||
== | = Conclusions = | ||
It has long been clear that valence plays a role in choice (Schneirla, 1959). Research on the role of arousal in choice is more recent (quantified for the first time in the present work), and unsurprisingly, less settled. Ariely and Loewenstein (2006) have demonstrated that arousal broadens the category of things “that are attractive.” Such a broadening could impact approach/avoidance behaviors. A related explanation is that arousal is associated with the salience of a stimulus. Salient events require immediate behavioral responses and therefore impact motivation (Cooper & Knutson, 2008). | |||
The best-fit model (behaviorally derived) and the current imaging work offers new insights regarding the mechanisms underlying affective choice. We believe that these insights have broad implications across many other fields in psychology. For example, neuroeconomists have suggested that various neural systems are involved in decision making, but little is known about how options with different values are compared with each other (Rangel, Camerer & Montague, 2008). The best-fit model provides a plausible ‘common currency’ to address these issues. | |||
Latest revision as of 19:05, 12 March 2012
Back to Psych 204 Projects 2009
Affect and Choice: Background Information
Imagine deciding which of two equally priced and equally appropriate pictures to buy for a home office. Faced with such a decision, people are thought to consult their feelings and ask, “How do I feel about each option?” (Schwarz & Clore, 2003). A choice between the two affective states is thought to determine which picture is chosen.
Now imagine deciding whether to invest in risky high-yield stocks or safer lower-returning bonds. This decision involves considerations that are not purely affective. However, a growing literature suggests that even in such cases affective choice may play a pivotal role in decision making. Indeed, it has been suggested that affect serves as the “common currency” of decision making (Peters, Västfjäll, Gärling & Slovic, 2006). Decision makers decompose complex thoughts into affective states (Mellers, 2000; Pfister et al., 2008) that are then compared with each other, as in the picture example.
Despite the centrality of affective choice to decision making, it is not at all clear how choices between two affective states are made. Our starting point in addressing this issue is a distinction between two fundamental dimensions of affect—valence and arousal. The first, valence, represents the pleasantness/unpleasantness of the affect. The second, arousal, represents the degree of activation associated with the affect. These dimensions capture most of the variance in self-reported mood ratings (Osgood, Suci & Tanenbaum, 1957; Russell 1980; Barrett & Russell, 1999). In addition, cross cultural (Larsen & Diener, 1992), neurobiological (Posner et al., 2009), and developmental data (Russell & Bullock, 1985) demonstrate that – as Wundt (1912) postulated – valence and arousal are core components of affective states. Valence and arousal are often drawn as perpendicular dimensions to create an affective 2-space (Figure 1).
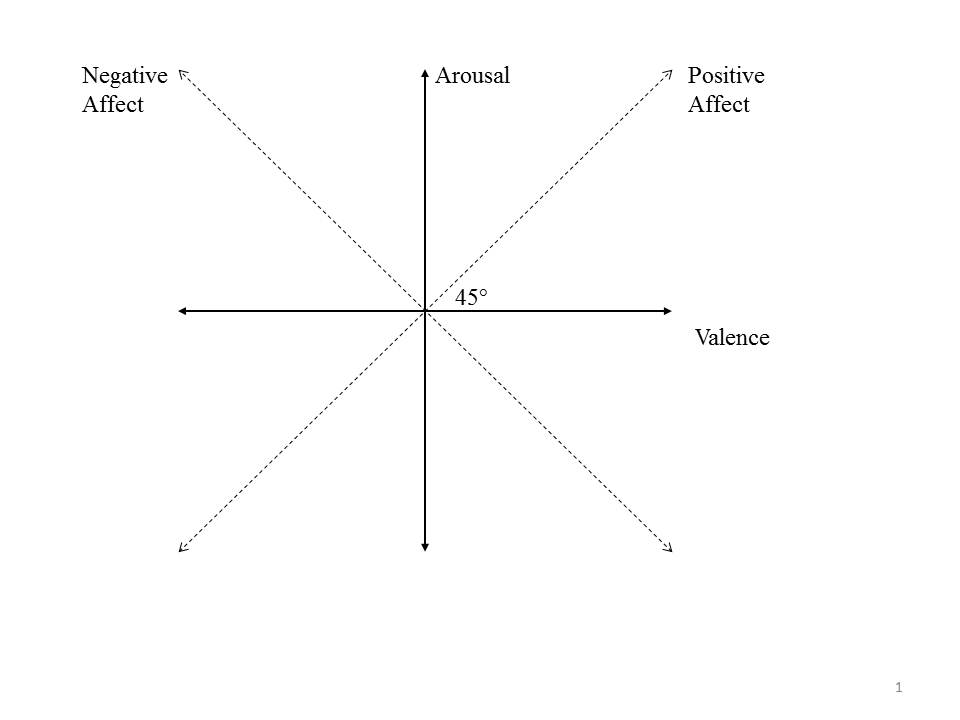
Experiment Structure
60 IAPS pictures (images with affective content) were split into 30 pairs. Within a pair, one picture was designated as Picture 1 and the other as Picture 2. Picture 1 and Picture 2 were counterbalanced for valence and arousal (IAPS population ratings).
In the fMRI, subjects were shown Picture 1 for 500ms followed by Picture 2 for 500ms. They were asked to make a choice between the two pictures. The chosen picture was displayed for 4s—a duration long enough to ensure that the preferred picture was viewed and processed; this attached real affective consequences to each choice.
Outside the fMRI, subjects were asked to rate each image on a 1-7 scale for valence and arousal
Hypothesis
We expected to see 2 sets of results:
1) Distinct areas of the brain would be active for high vs. low valence pictures and for high vs. low arousal pictures. Since a variety of independent sources confirm that valence and arousal are core dimensions of affect, finding neural bases that underlie these dimensions is central to the understanding of affect.
2) We expect activations in these regions to drive choice. In particular a greater positive arousal (PA) would predict choice in cases where both pictures have PA > 0. Similarly in cases where NA > 0, we expect that lower NA would predict choice.
fMRI Methods
Expected Bases of A/V
Based on prior work in the Knutson Lab [1] we expected particular sub-cortical areas to show differential activation:
For Positive Arousal (PA) we expected to see some activation in the Ventral Striatum (Nucleus Accumbens) in particular.
For Negative Arousal (NA) we expected to see some activation in the Insula.
In addition we expected to see some activation in the mPFC for both high valence and arousal pictures.
Subjects
Subjects were 5 healthy volunteers. Results presented here are for a single subject (myself)
MR acquisition
Data were obtained on the CNI scanner at Stanford University. Functional and Anatomicals were reconstructed using CNI algorithms.
AFNI was used to process and analyze the data.
Trial Structure & Shaving of Leading TRs
In this experiment we used the following presentation schedule:
Pic 1: 500MS followed by fixation for 500MS
Pic 2: 500MS followed by fixation for 500MS
Consider Your Choice: 2s
Make Your Selection: 2s
Variable ITI: 2/4/6s
Due to this Trial Structure we chose at TR = 1s.
Further we chose to have 8 leading and 8 trialing TRs to adjust for fMRI start up effects. We eliminated these TRs prior to pre-processing.
Pre-processing
All data were motion corrected, smoothed, drift corrected, normalized and warped.
Motion Corrected
The AFNI command 3dvolreg was used for motion correction.
Smoothing
A blurring kernel of 4mm was used.
Drift Correction
A Fourier highpass filter of 0.011 was used to drift-correct
Additionally:
Data was normalized to percent signal change Anatomical and functional scans were wrapped to talairach space
Post-processing
We used regressors and self ratings to do the analysis.
First we created contrasts (1 0 or 1 0 -1 or parametric) that described the effects.
1,0 -> picture on, picture off
1,0,-1 -> high valence picture, no picture, low valence picture (similarly for arousal)
3,2,1,0,-1,-2,-3 --> exact ratings of valence/arousal deviating from mean 0
Next we convolved the contrasts with the hemodynamic response function
Finally we took the normalized functional data and ran a regression with the contrasts (as long as all contrasts are orthogonal, or aka non-covariance between contrasts). We examined the contrasts in AFNI
Results
Valence
The ventral striatum was reliably activated for positively valenced pictures compared to negatively valenced pictures (Figure 2)
We also saw de-activation in the mPFC for highly valenced pictures -- indicating that low valenced pictures are cognitively processed to a greater extent than high valenced pictures (Figure 3)
Expectedly we also observed insula activation for negatively valenced pictures (Figure 4)
Arousal
There were no reliable sub-cortical activations for arousal. However we did observe PFC and mPFC activations for low arousal pictures. (Figure 5)
Choice
We do observe that images with higher NAcc activation are chosen over images with lower NAcc activation.
Similarly, negative images with lower insula activation are chosen over images with higher insula activation.
Conclusions
It has long been clear that valence plays a role in choice (Schneirla, 1959). Research on the role of arousal in choice is more recent (quantified for the first time in the present work), and unsurprisingly, less settled. Ariely and Loewenstein (2006) have demonstrated that arousal broadens the category of things “that are attractive.” Such a broadening could impact approach/avoidance behaviors. A related explanation is that arousal is associated with the salience of a stimulus. Salient events require immediate behavioral responses and therefore impact motivation (Cooper & Knutson, 2008). The best-fit model (behaviorally derived) and the current imaging work offers new insights regarding the mechanisms underlying affective choice. We believe that these insights have broad implications across many other fields in psychology. For example, neuroeconomists have suggested that various neural systems are involved in decision making, but little is known about how options with different values are compared with each other (Rangel, Camerer & Montague, 2008). The best-fit model provides a plausible ‘common currency’ to address these issues.