Thermal Imaging and Pitvipers
Introduction
Electromagnetic radiation refers to traveling oscillations in the electric and magnetic fields. The name “electromagnetic radiation” has about nine syllables too many, so in physics it is often simply called “light”. Light can be categorized based on the portion of the electromagnetic spectrum it resides in (e.g. “visible light”) or it can be categorized based on its source (e.g. “laser light”). In this report we will refer to two regions of the spectrum - the “visible” and the “infrared”. The visible region is the portion our eyes can see and consists of wavelengths 400 - 700 nm. The infrared region is everything between the visible and microwave portions of the spectrum, or wavelengths roughly from 700 nm - 1 mm. We will focus on light generated by the thermal motion of particles, which we call “thermal light”.
Although different portions of the electromagnetic spectrum often are treated as completely separate, we will show here that both the visible and infrared are used by animals for vision. Our goal is to understand the components fundamental to imaging in these two wavelength regions and to connect the animals’ vision to today’s technology.
We first briefly introduce the origins of thermal light. Then we will discuss the evolution of vision in animals to both the visible and the infrared, before comparing the two and noting the implications for human camera technology in the two regions of the spectrum.
Background
Scientists observed that warm things emitted light, and then tried to model it. In 1900 Max Planck was shown data on the spectrum of thermal light, and the same day guessed a formula that modeled it. He started with the answer and then worked backwards to explain it, changing any physics that disagreed along the way - in doing so he invented the Boltzmann constant and also quantized the energy levels of the light - marking the very beginning of quantum mechanics [Hollandt 2012]. His equation for the spectrum of thermal light emitted by an object at temperature T is [Planck 1910]
![]()
There are no geometrical terms here - the object is assumed to be a “blackbody” that perfectly absorbs all incident light, and thus also perfectly emits thermal light. Most everyday objects are not perfect absorbers, but instead are partially reflective. We can model these “greybodies” by multiplying the blackbody spectrum by the emissivity - a number between 0 and 1 that represents how absorptive or emissive the object is. In general, the emissivity depends on wavelength and angle, but oftentimes an average emissivity is sufficient.
By integrating the greybody intensity spectrum we find that the power of thermal radiation scales with temperature to the fourth power times the emissivity.
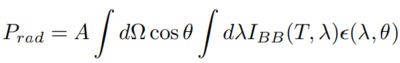
![]()
![]()
![]()
To summarize, everything emits thermal light, with a spectrum and radiance that depends on material and temperature. The sun has a temperature of about 6000 K and emits light with a peak wavelength in the visible around 500 nm. Everything at earth-scale temperatures near 300 K emits in the infrared with a peak wavelength around 10 um.
Methods
Results
Conclusions
References
Appendix
You can write math equations as follows:
You can include images as follows (you will need to upload the image first using the toolbox on the left bar, using the "Upload file" link).


