Natalie
Back to Psych 204 Projects 2009
Background
Major depressive disorder (MDD) affects nearly 20% of all Americans and is the most common of all psychiatric disorders (Kessler & Wang, 2009). MDD affects about 9% of all US children and adolescents (Avenevoli, 2008), significantly altering the developmental trajectory of millions of Americans. Not only does this disorder affect individuals’ development, but it also continues later into life; in fact, 40% of depressed adolescents have a subsequent episode of depression within three years (Kessler et al., 2001), and most depressed individuals experience recurrent episodes throughout adulthood (Lewinsohn et al., 1998; Rao, Hammen, & Daley, 1999; Weissman et al., 1999). Many negative consequences of early onset depression persist into adulthood, including impairments in academic and occupational performance, interpersonal relationships, quality of life, physical well-being, and reports of life satisfaction (Lewinsohn et al., 2003). These findings clearly highlight the need to gain a more comprehensive and complete understanding of the etiology and course of this disorder in adolescence.
Early onset of depression in adolescence is of particular importance because of the emotional lability that characterizes this stage of development (Arnett, 1999). Adolescents typically report experiencing more extreme emotions (particularly negative) than do both younger children and adults (Larson & Richards, 1994). The results of neuroimaging studies provide a plausible mechanism for this emotional reactivity in adolescence: heightened subcortical limbic activation and attenuated prefrontal cortex regulation in response to emotional stimuli (Galvan et al., 2006; Guyer et al., 2008; Monk et al., 2003). This imbalance between matured emotional processing in the limbic system and an underdeveloped pre-frontal cognitive control system may underlie a number of typical adolescent behaviors such as risk-taking and emotional reactivity. For most adolescents, however, this imbalance in neural systems does not result in a clinical life-long disorder. Therefore, it is imperative that we systematically examine these systems, both behaviorally and neurologically, in order to understand the departure from typical adolescent development that predisposes individuals to MDD.
One mechanism of cognitive control that may contribute to negative attention biases observed in depressed individuals is an impairment in inhibitory functioning. The difficulty experienced by depressed individuals in inhibiting negative or sad material in the environment, combined with their selective attention to negative stimuli, can lead to alterations in mood state and negative affect, initiating or sustaining a depressive episode (Joormann, Yoon, & Zetsche, 2007). If inhibitory dysfunction is a critical factor in the etiology and maintenance of rumination and negative mood states, then differences between individuals with and without MDD should be apparent in cognitive inhibition tasks, such as the Go/No-Go task (GNG). The GNG task requires the participant to respond to a selected “go” target, presented more than 75% of the time, while overriding the prepotent response when presented with the “no-go” target (Casey et al., 1997). Given the difficulties of depressed youth in inhibiting the processing of negative stimuli, the GNG task can be adapted to examine the inhibition of emotionally salient stimuli.
Prior research suggests that inhibitory function has a protracted development throughout childhood, with individuals not reaching full capacity on inhibitory tasks, such as the Stroop, flanker, or classic GNG tasks until adolescence (Levin, et al., 1991). Researchers have attributed this impaired task performance in childhood to more extensive activation in the dorsal and lateral prefrontal cortex (PFC), suggesting that children are less efficient than are adults and need to activate more cortex in order to achieve the same level of cognitive control (Casey et al., 1997; Durston et al., 2002). Adolescents have been found to differ significantly from both children and adults in their performance emotional GNG tasks. Hare et al. (2008) examined inhibitory control in these three age groups as participants completed a GNG task with fearful, happy, and calm facial expressions as targets and non-targets. They found increased amygdala activation in adolescents relative to both children and adults, supporting the formulation that adolescence is a period during which there is immature top-down control over more mature bottom-up emotional processing. Furthermore, Hare et al. also found a negative correlation between a self-report measure of trait anxiety and activity in the ventral PFC, suggesting the subcortical limbic system and prefrontal executive control systems play a role in the development of affective symptoms.
To date, no studies have examined the neural components of inhibitory processing during an emotional GNG task in adolescents who are currently experiencing a major depressive episode. In the present study we will use an emotion-modified GNG task to examine the neural correlates of inhibitory behavior in adolescents diagnosed with MDD. Either an emotional face or an emotionally neutral tree will be presented for 1 s before the target, allowing for an examination of the effects of emotional processing on inhibitory control. Presenting the emotional face prior to the response target will permit the separation of the effects of emotional processing from those of inhibitory control.
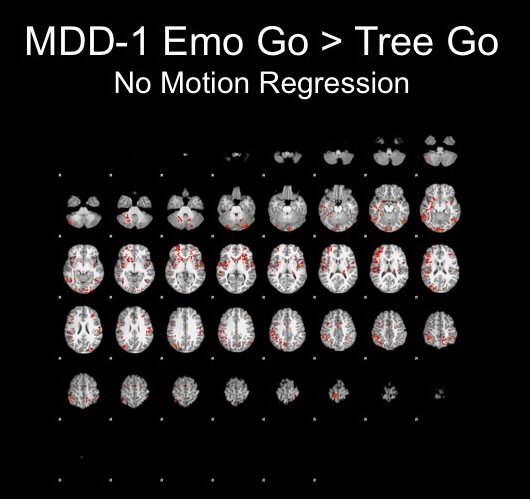
For the purposes of this project, I am interested in the effects that motion and motion correction have on the results of my experiment. Therefore, I will examine two subjects, one with minimal motion and one with an extreme amount of motion, and attempt to correct this motion with multiple methods. I will use the contrast Emotional Go > Tree Go in order to use the presence of the FFA as a signal of effective motion correction in the data.
Methods
Task Design
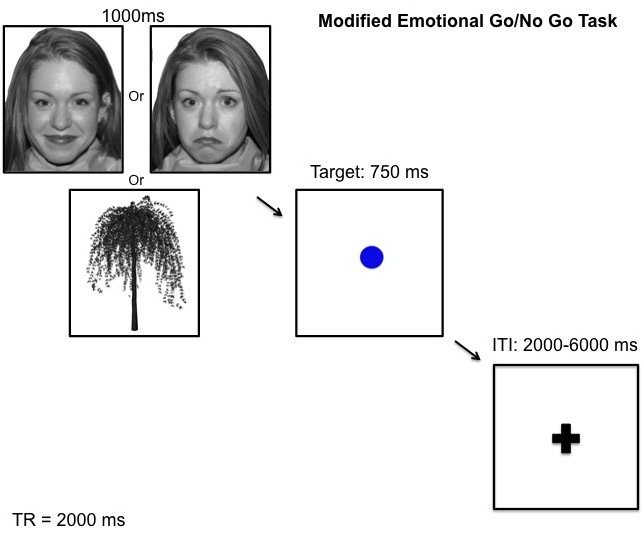
The modified GNG task requires participants to make a button press when they see the visual target “go” cue (a blue circle) and inhibit this response when they see the infrequently presented “no-go” cue (a blue square). The target cue is presented approximately 70% of the time, creating a prepotent response tendency that must be inhibited in the presence of the “no-go” cue. In order to assess inhibitory function in the presence of emotional stimuli, we modified the traditional GNG task by presenting either an emotional face picture or a neutral tree picture immediately before the target or non-target cue.
Subjects
Subjects were 2 teens with MDD, 1 control teen.
MR acquisition
All scans will be conducted on a 3 Tesla GE whole-body scanner at Stanford’s Lucas Center. Foam padding was used to minimize head movement. Twenty-nine axial slices were taken with 4-mm slice thickness. High-resolution T2-weighted fast spin echo structural images (TR=3000ms, TE=68ms, ETL=12, FOV=22cm, 192x256) were acquired for anatomical reference. A T2*-sensitive gradient echo spiral in/out pulse sequence were used for functional imaging (TR =2000ms, TE=30ms, flip angle=80°, FOV=22cm, nframes=280, disdaqs=4, scan time=9.26, 4 repeats). An automated high-order shimming procedure, based on spiral acquisitions, was used to reduce B0 heterogeneity. Additional high-resolution images were acquired with an axial 3D FSPGR sequence for T1 contrast (140 slices, 1.3mm thickness, TR=6.0ms, TE=1.2ms, TI=500ms, flip angle 11°, FOV=24cm, 192x256). The axial 3D FSPGR image acquisition were repeated and the two scans were averaged after image coregistration off-line to improve SNR.
MR Analysis
The MR data was analyzed using fsl (http://www.fmrib.ox.ac.uk/fsl/).
Pre-processing
All data were motion corrected (MCFLIRT), skull stripped (BET) and a high pass filter was performed on all functional data. The four task runs were combined in a fixed effects model. All contrasts below are thresholded at Z=1.7, uncorrected for multiple comparisons.
Methods of Motion Correction
On the first subject with minimal motion, I explored the effect of adding the 6 motion parameters (x,y,z,pitch,yaw,roll), to the design matrix, as nuisance regressors.
On the second subject with an inordinate amount of motion, I not only attempted to correct for motion by regressing out the 6 motion parameters, but also attempted to remove spikes by removing the affected volumes, and also tried to correct for motion spikes by adding nuisance regressors where those spikes occurred.
All contrasts below at of the emotional go condition > neutral tree condition in order to use the FFA as a benchmark for the success of motion correction.
Results
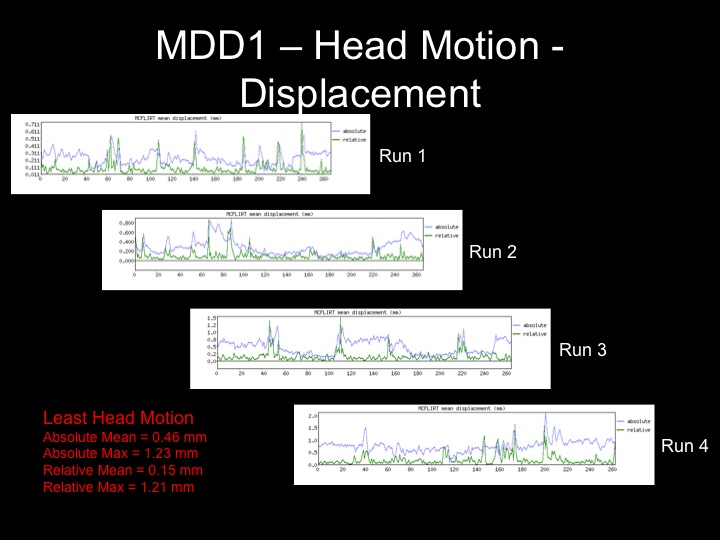
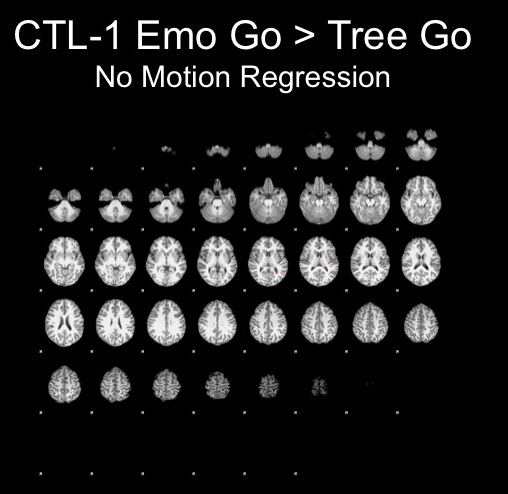
Fixed Effects GLM Results for a Subject with Minimal Motion
Head displacement across the timecourse for 1 subject:
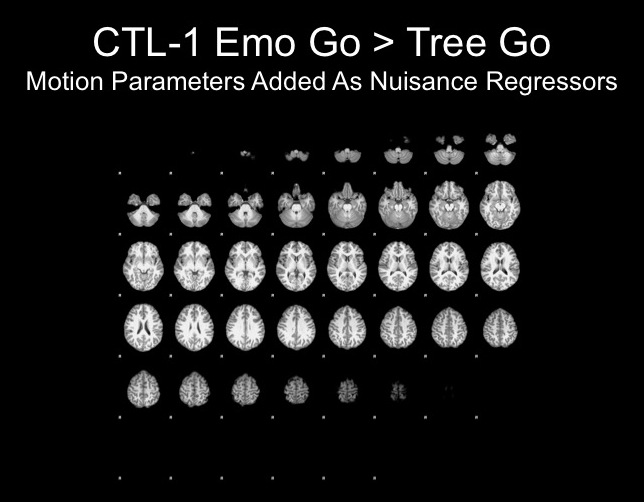
Results with and without motion parameters as nuisance regressors:
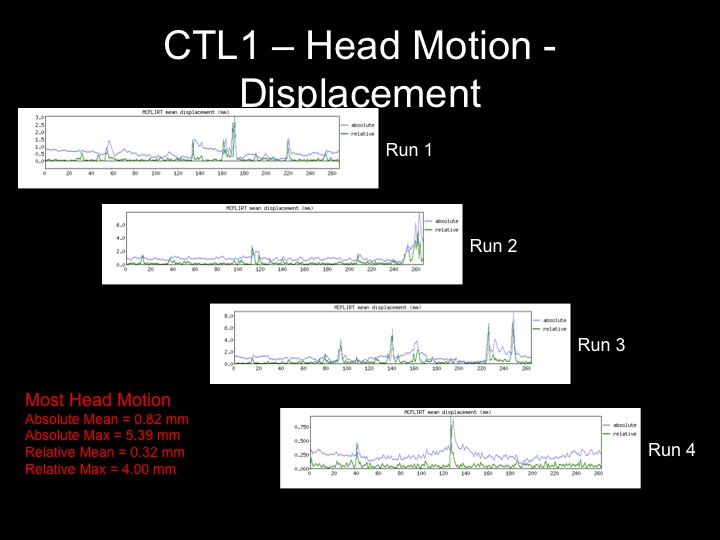
Fixed Effects GLM Results for a Subject with Significant Motion
Head displacement across the timecourse for 1 subject:
GLM Results with and without motion parameters as nuisance regressors:
These results were very discouraging and showed no sign of FFA activation, so I decided to look closer at each run and attempt to correct for the motion.
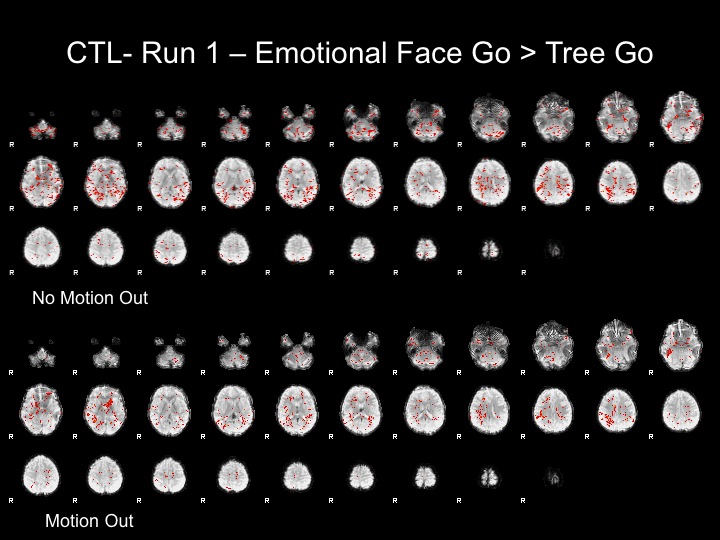
Run1
The motion for this subject was not terrible and therefore, I decided that using the motion parameters as regressors would suffice here.
The motion parameters helped by removing noise, however, this may be task-related activation, therefore further analyses are necessary to determine whether or not this is the best step.
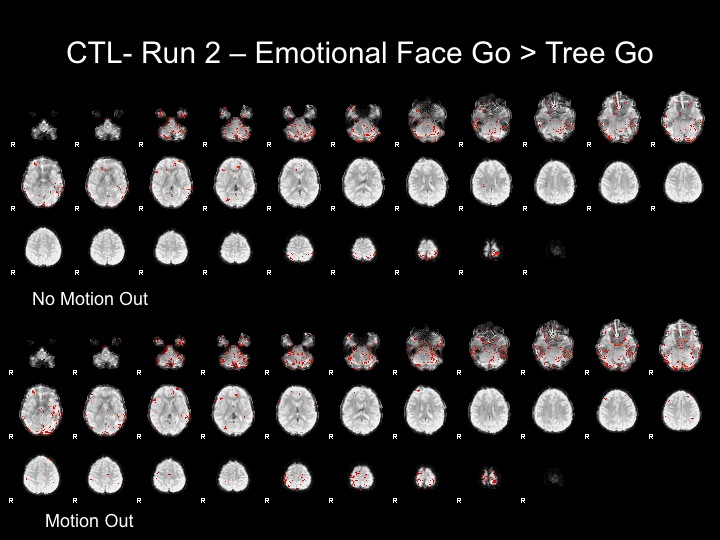
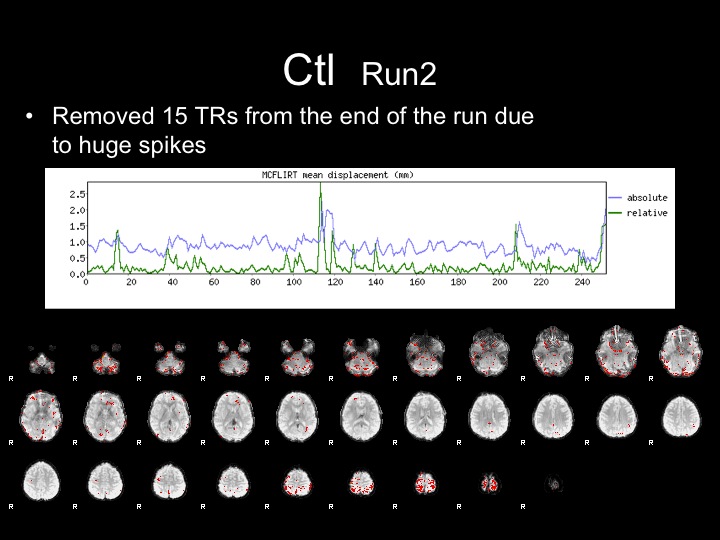
Run2
This subject has significant spikes towards the end of the run, so further analyses are necessary to remove the effects of motion.
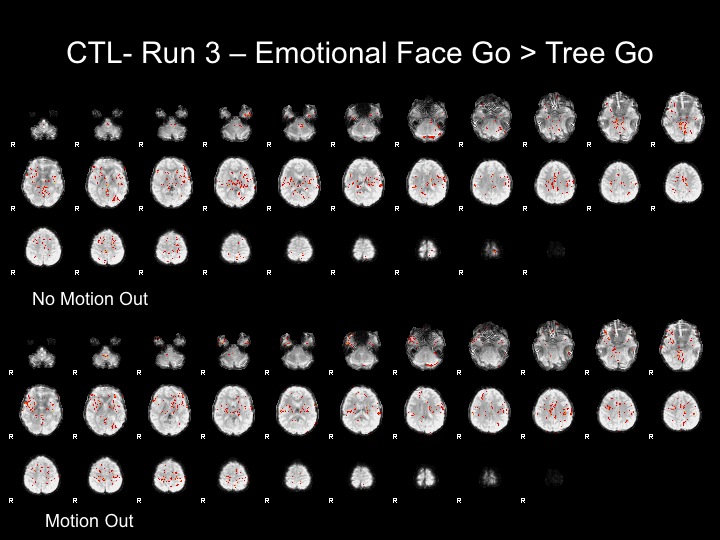
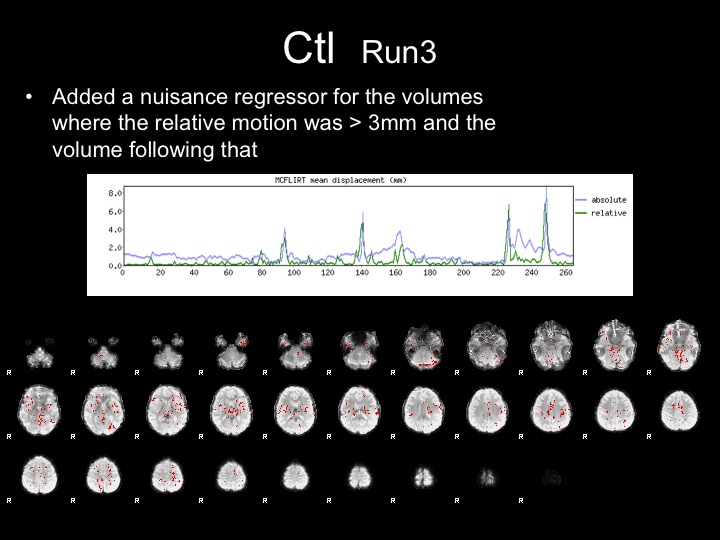
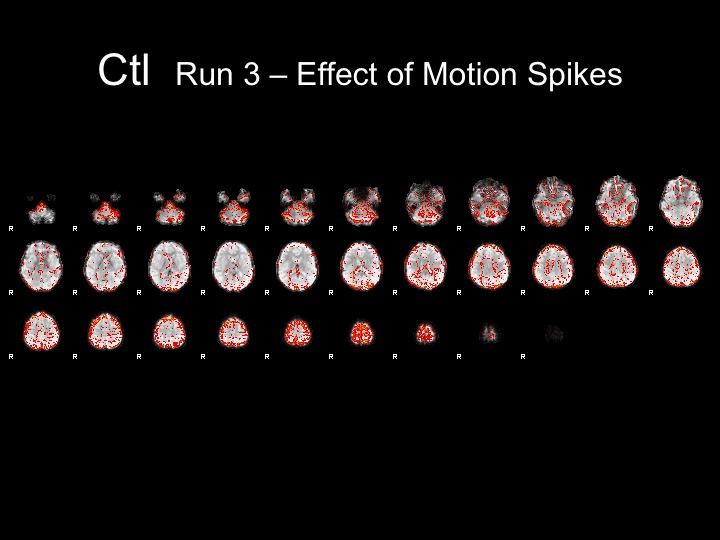
Run3
This subject has significant spikes in the middle of the run, so further analyses are necessary to remove the effects of motion.
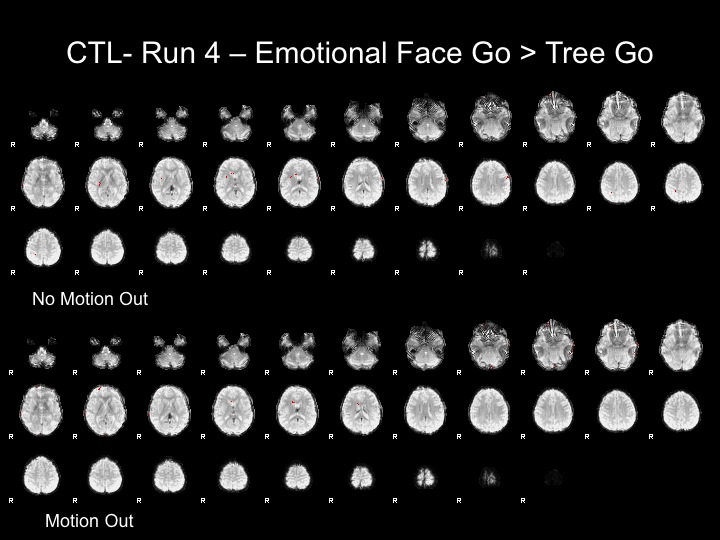
Run4
The motion for this subject was not terrible and therefore, I decided that using the motion parameters as regressors would suffice here.
After adding motion regressors to these maps, there is still very little activation. This may be due to the subject's inattention, as this is the end of a 35 minute task. However, there could be multiple reasons that this subject does not show significant activation.
Simple Model GLM Results for a Subject with Significant Motion using "Motion Corrected" Data
Run2-Motion Volumes Removed
With this run, I attempted to correct for the effects of motion by removing the last 15 TRs from the raw dataset, and proceeded with preprocessing and GLM analyses.
Removing these movement TRs helped the analyses, but did not completely remove the effects of motion from the statistical maps and FFA is not present in these maps.
Run3-Motion Spikes Regressed Out
With this run, I attempted to correct for the effects of motion by adding nuisance regressors to remove the variance associated with the motion spikes.
Adding these regressors did not completely remove the effects of motion from the statistical maps and FFA is not present in these maps.
'The Variance Contributed by Motion Spikes'
Fixed Effects Model with all 4 Runs w/ Motion Correction
Conclusions
Using motion parameters as regressors can help clean up your data
However, using any kind of motion correction cannot salvage a subject with excessive motion (>3 mm for this project)
References
Kessler, R. & Wang, P. The Epidemiology of Depression. The Handbook of Depression. New York: Guilford; 2009.
Kessler, R.C., Avenevoli, S. & Merikangas, K.R. (2001). Mood disorders in children and adolescents: an epidemiologic perspective. Biological Psychiatry, 49, 1002-1014.
Lewinsohn PM, Rohde P, Seeley JR. (1998). Treatment of adolescent depression: frequency of services and impact on functioning in young adulthood. Depression and Anxiety. 7(1), 47-52.
Rao, U., Hammen, C. & Daley, S.E. (1999). Continuity of depression during the transition to adulthood: A 5-year longitudinal study of young women. Journal of the American Academy of Child and Adolescent Psychiatry, 38, 908-015.
Weissman, M.M., Wolk, S., Goldstein, R.B., Moreau, D., Adams, P., Greenwald, S., Klier, C.M., Ryan, N.D., Dahl, R.E., Wickramaratne, P. (1999). Depressed adolescents grown up. Journal of the American Medical Association, 281, 1707-1713.
Lewinsohn, P.M., Rohde, P., Seeley, J.R., Klein, D.N. & Gotlb, I.H. (2003). Psychosocial functioning of young adults who have experienced and recovered from major depressive disorder during adolescence. Journal of Abnormal Psychology, 112(3), 353-363.
Arnett, J.J. (1999). Adolescent storm and stress, reconsidered. American Psychologist, 54(5), 317-326.
Larson, R. & Richards, M.H. (1994). Divergent realities: The emotional lives of mothers, fathers, and adolescents. New York: Basic Books.
Galvan, A. Hare, T.A. Parra, C.E., Penn, J., Voss, H., Glover, G., Casey, B.J. (2006). Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behavior in adolescents. Journal of Neuroscience, 26, 6885-6892.
Guyer, A.E., Monk, C.S., McClure-Tone, E.B., Nelson, E.E., Roberson-Nay, R., Adler, A.D., Fromm, S.J., Liebenluft, E., Pine, D.S. & Ernst, M. (2008). A developmental examination of amygdala response to facial expressions. Journal of Cognitive Neuroscience, 20(9), 1565-1582.
Monk, C.S., McClure, E.B., Nelson, E.E., Zarahn, E., Bilder, R.M., Leibenluft, E., Charney, D.S., Ernst, M. & Pine, D.S. (2003). Adolescent immaturity in attention-related brain engagement to emotional facial expressions. NeuroImage, 20, 420-428.
Joormann, J., Talbot, L. & Gotlib, I.H. (2007) Biased processing of emotional information in girls at risk for depression. Journal of Abnormal Psychology, 116(1), 135-143.
Casey, B.J., Trainor, R.J., Orendi, J.L., Schubert, A.B., Nystrom, L.E., Giedd, J.N., Castellanos, F.X., Haxby, J.V., Noll, D.C., Cohen, J.D., Forman, S.D., Dahl, R.E. & Rapoport, J.L. (1997). A developmental functional mri study of prefrontal activation during performance on a go-no-go task. Journal of Cognitive Neuroscience, 9(6), 835,847.
Durston, S., Thomas, K.M., Yang, Y., Ulug, A.M., Zimmerman, R.D. & Casey, B.J. (2002). A neural basis for the development of inhibitory control. Developmental Science, 5(4), F9-F16.
Hare, T.A., Tottenhan, N., Galvan, A., Voss, H.U., Glover, G.H. & Casey, B.J. (2008). Biological substrates of emotional reactivity and regulation in adolescence during an emotional go-nogo task. Biological Psychiatry, 63, 927-934.